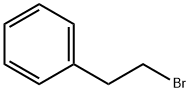
(2-Bromoethyl)benzene:Application and Preparation
General Description
(2-Bromoethyl)benzene is an inhibitor of histone demethylase, an amine compound having anticancer activity and a therapeutic agent for eye disorders. It is an industrially extremely useful compound as a pharmaceutical intermediate and a synthetic intermediate of a functional monomer such as sodium parastyrene sulfonate.[1]

Figure 1 Solution of the (2-Bromoethyl)benzene
Preparation
There are two methods for producing (2-Bromoethyl)benzene. For example:First method for producing (2-Bromoethyl)benzene using an LED with a wavelength of 365nm as a light source.Four 500 ml flasks to which a cooling tube, a temperature insertion tube, and two PTFE tube adapters are attached. The flask was charged with 30.0g of heptane under a nitrogen atmosphere. A tube for supplying a hydrogen bromide gas and a separately prepared raw material mixed solution was attached to each adapter. The tube for supplying the hydrogen bromide gas was installed so that the tip was immersed in the reaction solution, and the tube for supplying the raw material solution was installed so as to drip into the reactor. A spot irradiator was installed at a position where the light intensity hitting the side surface of the reactor was 300mW/cm2 (543.6mW/cm2 for a feed flow rate of 1ml/min of styrene only), and light irradiation was started. At the same time as the start of light irradiation, the supply of hydrogen bromide gas and the raw material solution was started. The respective flow rates were 140.0ml / min for hydrogen bromide gas and 2.1ml / min for the raw material solution. Two hours after the start of the feed, the supply of hydrogen bromide gas and the raw material solution was stopped, and light irradiation was continued for 30 minutes to react the remaining styrene. Then, nitrogen was blown into the reaction solution at a flow rate of 500ml/min for 1 hour to perform a bubbling operation, and hydrogen bromide gas in the reaction solution was removed. 60ml of water was added to the reaction solution after the bubbling operation, the solution was separated, the organic layer was heated and concentrated, and heptane was distilled off.Finally, (2-Bromoethyl)benzene was gained.[1]
Second method for producing(2-Bromoethyl)benzene using light having a wavelength of 365nm in a mercury lamp as a light source.A cooling tube, a temperature insertion tube, and two PTFE tube adapters were attached. 25.0g of heptane was charged into a 500ml four-necked flask under a nitrogen atmosphere. A tube for supplying a hydrogen bromide gas and a separately prepared raw material mixed solution (styrene 50.0g, heptane 105.0g) was attached to each adapter. The tube for supplying the hydrogen bromide gas was fed while being immersed in the reaction solution, and the tube for supplying the raw material mixed solution was installed so as to drip into the reactor. A light source device (with a 365nm bandpass filter attached) was installed at a position where the light intensity hitting the side surface of the reactor was 2mW/cm2 (10.9mW/cm2 for a feed flow rate of 1ml/min of styrene only), and light irradiation was started. Then, the supply of hydrogen bromide gas at a flow rate of 47 ml/min and the raw material mixed solution at a flow rate of 0.7ml/min was started. After 5 hours from the start of the feed, the supply was stopped and light irradiation was continued for 30 minutes to react the remaining styrene. After the irradiation was stopped, nitrogen was blown into the reaction solution at a flow rate of 100ml / min for 1 hour to perform bubbling, and hydrogen bromide gas in the reaction solution was removed. 60ml of pure water was added to the reaction solution after the bubbling operation, the solution was separated, the organic layer was heated and concentrated, and heptane was distilled off and (2-Bromoethyl)benzene was gained.[1]
Application
The (2-Bromoethyl)benzene is extremely useful in industry.[1]For instance, (2-Bromoethyl)benzene may be employed as a starting material in a number of organic syntheses. It may be saponified in order to produce phenylethyl alcohol.[2](2-Bromoethyl)benzene was used as raw material, Condensation reaction was happened between those mol, come into being 1-phenethyl-1H-imidazole.[3]Additionally, Using (2-Bromoethyl)benzene as initiator can prepare branched poly (styrene-co-acrylonitrile) via atom transfer radical polymerisation.[4]
Reference
[1]Maedera, Satoshi, Shigeta, Yusuke.Method for producing high-purity β-bromoethylbenzene[P].JP2021130650.2021,09,09.
[2]Fluchaire M, Javorski S, et al. Process of preparing beta-bromethyl benzene[P]. US2082946A.1937.
[3]Nie G, et al.Thesynthesis research of 1-phenethyl-1H-imidazole[J].Shandong Huagong, 2020, 49, (18):17-20.
[4]Huang W, Xue X, Chen J, et al. Preparation and characterisation of branched poly (styrene-co-acrylonitrile) via atom transfer radical polymerisation using β-bromoethyl benzene as initiator[J]. Materials Research Innovations, 2014, 18(3):214-219.
Related articles And Qustion
See also
Lastest Price from (2-Bromoethyl)benzene manufacturers

US $0.00/KG2026-01-31
- CAS:
- 103-63-9
- Min. Order:
- 1KG
- Purity:
- ≥98.5%
- Supply Ability:
- 80 Tons/Month
US $0.00-0.00/KG2025-11-28
- CAS:
- 103-63-9
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS